Abstract
Background: Standard first-line (1L) intensive induction chemotherapy (IC) has often been based off of a 7+3 (cytarabine + anthracycline-based chemotherapy) based backbone, on which approximately 60%-70% of adults with AML achieve complete remission (CR). Newer therapies such as venetoclax (VEN) + a hypomethylating agent (HMA) have demonstrated durable responses and promising efficacy outcomes; however, few RW studies have examined outcomes comparing IC, VEN-based, and other lower intensity therapies. This study aimed to compare AML induction regimen treatment patterns based on patient characteristics and outcomes based on the following 3 different therapies; IC; a VEN-based therapy (eg, VEN monotherapy or VEN + decitabine, azacitidine, or low-dose cytarabine [LDAC]); or a non-VEN-based low-intensity therapy (ie, single-agent HMAs, glasdegib + LDAC, or HMA + LDAC [excluding single-agent LDAC or hydroxyurea]).
Methods: A retrospective, observational, United States-based, multisite cohort study utilizing electronic health record data from patients (aged ≥18 years) with newly diagnosed AML who initiated 1L IC between Jan 1, 2016 and Dec 31, 2019, 1L VEN-based therapy between Jan 1, 2019 and Dec 31, 2020, or 1L non-VEN-based low-intensity therapy between Jan 1, 2019 and Dec 31, 2020 was conducted. Eligible patients had ≥6 months of follow-up after 1L initiation, unless <6 months of follow-up was available due to death during this interval and AML therapy not received as part of a clinical trial. Patients were categorized as achieving remission if they had a physician-reported best response of CR with no minimal residual disease (CRMRD−), CR, or CR with incomplete hematological improvement (CRi).
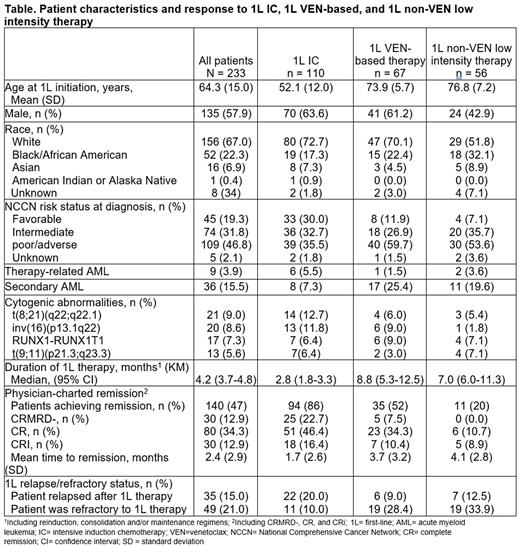
Results: Among 233 eligible patients, 58% were male, 67% were White, 4% had therapy-related AML, and 16% had secondary AML (Table). At AML diagnosis, 36% of the 1L IC cohort, 60% of the 1L VEN-based therapy cohort, and 54% of the 1L non-VEN-based low-intensity therapy cohort had adverse-risk disease (based on NCCN® risk stratification). Mean age at 1L initiation was 52 years, 74 years, and 77 years in the IC, VEN-based, and non-VEN-based low-intensity therapy cohorts, respectively. The most prevalent cytogenetic abnormalities were t(8;21)(q22;q22.1) (13%) and inv(16)(p13.1q22) (12%) in the 1L IC cohort, RUNX1-RUNX1T1 (9%) and inv(16)(p13.1q22) (9%) in the 1L VEN-based therapy cohort, and RUNX1-RUNX1T1 (7%) and t(9;11)(p21.3;q23.3) (7%) in the 1L non-VEN-based low-intensity cohort. The median duration of 1L therapy was 3, 9, and 7 months in the 1L IC cohort, 1L VEN-based therapy cohort, and 1L non-VEN-based low-intensity therapy cohort, respectively. In the 1L IC cohort, 86% of patients achieved remission during 1L treatment with 23%, 46%, and 16% achieving CRMRD−, CR, and CRi responses, respectively. In the 1L VEN-based therapy cohort, 52% of patients achieved remission during 1L treatment with 8%, 34%, and 10% achieving CRMRD−, CR, and CRi responses, respectively. In the 1L non-VEN low-intensity therapy cohort, 20% of patients achieved remission during 1L treatment with 0%, 11%, and 9% achieving CRMRD−, CR, and CRi responses, respectively. Among those achieving remission, the median time to remission was 1, 3, and 6 months in the 1L IC, 1L VEN-based therapy, and 1L non-VEN-based low-intensity therapy cohorts. In the 1L IC cohort, 20% of patients relapsed after 1L therapy and 10% of patients were refractory. In the 1L VEN-based therapy cohort, 9% of patients relapsed after 1L therapy and 28% of patients were refractory. In the 1L non-VEN based low intensity therapy cohort, 13% of patients relapsed after 1L therapy and 34% of patients were refractory.
Conclusions: As anticipated, patients who received 1L IC had the youngest median age at 1L initiation. More than half of the patients who received 1L VEN-based therapy and one-fifth of the patients who received 1L non-VEN based low-intensity therapy in this RW cohort study achieved remission; patients who received 1L IC had the highest rate of remission and the shortest time to remission. Additionally, patients who received 1L IC had the lowest rate of refractory disease across all the treatment cohorts assessed. Further research is warranted to determine appropriate treatment approaches for patients with AML based on treatment effectiveness, patient medical history, and IC eligibility.
Disclosures
Maher:BMS: Research Funding. Brown-Bickerstaff:Cardinal Health: Current Employment. McBride:Bristol Myers Squibb: Current Employment. Gajra:Cellectar: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Cardinal Health: Consultancy, Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Liassou:Cardinal Health: Current Employment. Qiu:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Garretson:Cardinal Health: Current Employment. Laney:Cardinal Health: Current Employment. Huggar:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Hughes:Amgen: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy; Rigel Pharmaceuticals: Other: grants and contracts; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal